These desperate efforts ask parents to overcome nearly impossible obstacles. They must become experts in drug development, raise millions, and tirelessly cajole scientists. Few people can pull it off.
“There are a lot of people who know how to do gene therapy, but the knowledge is all fragmented, and so much can go wrong,” says Sanath Kumar Ramesh, a software developer whose son is afflicted by a different rare disease. Ramesh founded an organization, Open Treatments, that is building software families can use to organize gene-therapy research, including steps such as hiring scientists to create animal models of an illness.
“I think in the future, the distinction between scientists and parents is going to be blurred,” he says.
For parents whose kids have already been accepted into the Dayton trial, gene therapy may be their last chance. One of them is Meagan Rockwell, a nail technician in Cedar Rapids, Iowa, whose daughter, Tobin Grace, now three and half, was diagnosed with Canavan in 2018.
“They told us sorry, there is nothing we can do—no treatment, no cure—you will be lucky if she sees her fifth birthday. It was a hard blow, to know your only child has a life-limiting brain disease,” Rockwell says.
Rockwell says she found out about Leone’s gene-therapy effort online and eventually raised more than $250,000. “At the time, Tobin was the youngest person in the US with Canavan, and I think that played a huge factor in her acceptance,” she says, adding that Leone tells parents money puts them at the front of the line but doesn’t guarantee treatment.
Bateman-House, the bioethicist, says another risk is whether parents can really judge the benefits of an experimental procedure in a “dispassionate” way, especially if they have sunk a fortune into the effort. “It’s not only that their child is facing a dangerous condition; it’s that their blood, sweat, and tears is what is funding this intervention,” she says. “It could be incredibly difficult for a parent to change their mind and say ‘We are not going to do this.’“
Hope versus risk
The Dayton study currently has enough supplies of the genetic drug to treat only nine or 10 children. It was manufactured in Spain, but only after the researchers and families overcame what they call an ordeal of red tape, delays, and obstacles, some thrown up by government regulators who decide which genetic treatments can be tried and whether trials are properly planned.
At one point, in 2019, the Landsmans took their sons to the US Food and Drug Administration for a meeting they landed after dozens of calls to lawmakers. “Beforehand we were a case number in their big pile of paper,” says Jennie Landsman, the boys’ mother. “They had very technical objections. In the meeting we held up Benny and Josh, and we said ‘We hope this issue that is so technical isn’t going to stop the treatment.’”
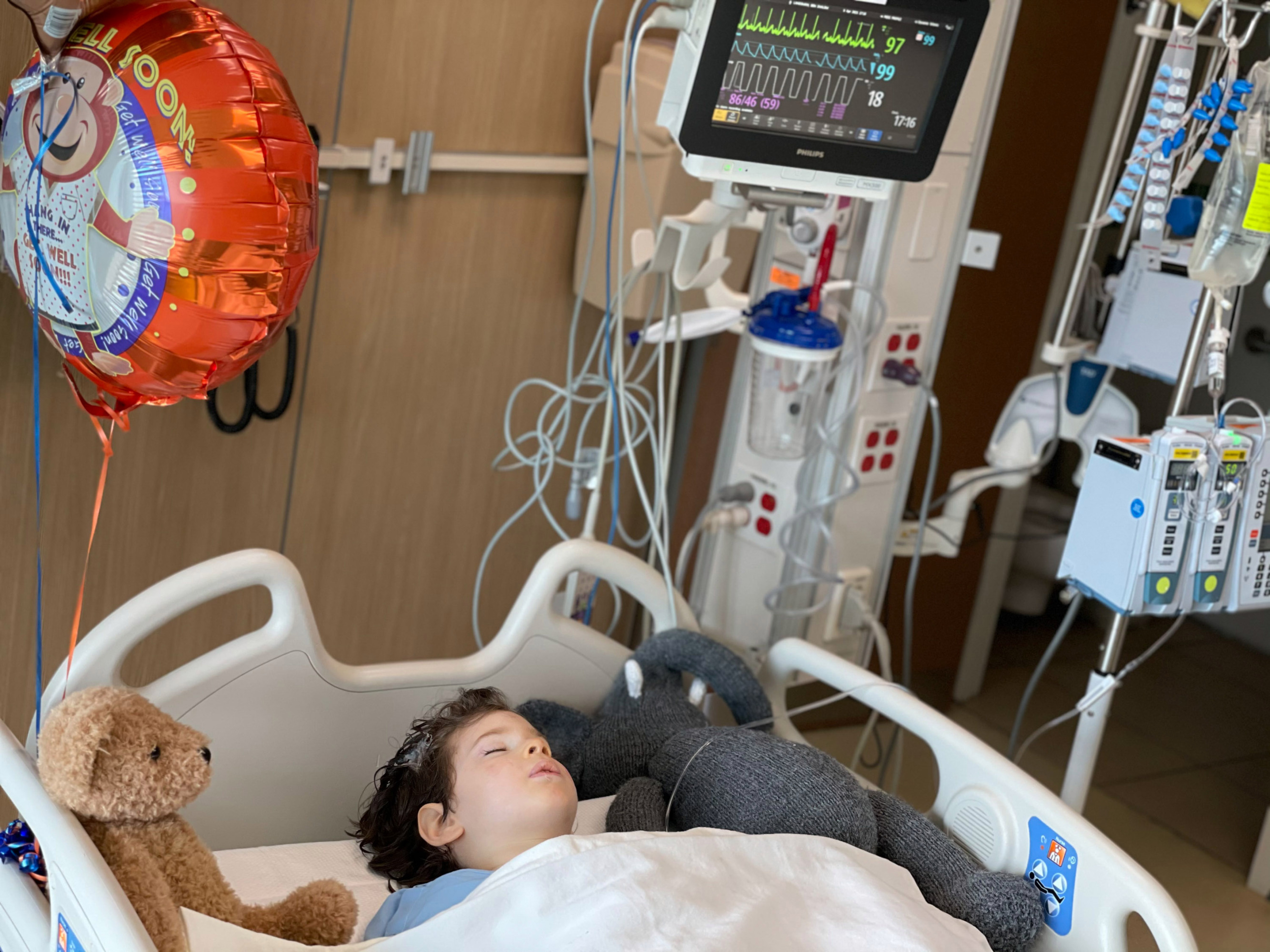
COURTESY OF JENNIE LANDSMAN
The Dayton trial won a greenlight in December and began barely in time for Benny, who will hit the age cutoff of five years in June. “Benny is the pilot. Benny is the ‘God, we hope this works’ kid,” says Rockwell, who doesn’t yet have a date for her daughter’s procedure.
What’s the chance the therapy works? Gene-replacement techniques have been having notable successes, curing kids who don’t have immune systems, and preventing brain diseases. Since 2017, a small number of gene therapies have also been approved for sale in the US, at prices as high as $2.1 million per child.
Record prices have stoked interest among specialist biotech companies, which now see a business even in super-rare diseases. One, called Aspa Therapeutics, says it has plans to initiate a different Canavan gene-therapy trial. Its CEO, Eric David, estimates there are 1,000 children alive with the disease in the US and Europe. “That, for us, is enough,” he says.
There’s no certainty gene therapy will succeed in Canavan. Even if the corrected gene stops the disease from progressing, the kids’ brains may have already been irreversibly damaged.